Featured Links
Featured Links
Featured Links
Featured Links
Primary
-
- Find Care
-
- Visitor Information
- Find a Location
- Shuttles
- Visitor Policies
-
-
-
- Our Virtual Care Options
- Virtual Urgent Care
- Virtual Visits for Primary & Specialty Care
- Online Second Opinions
- Participate in Research
-
- Contact us
-
- For Innovators
- Commercialization Guide for Innovators
-
-
- Research News
- Alzheimer's Disease
- Artificial Intelligence
-
- Overview
-
- Overview
- Getting Started
- New to Mass General Brigham
- International Patient Services
- What Is Patient Gateway?
- Planning Your Visit
- Find a Doctor (opens link in new tab)
- Appointments
- Patient Resources
- Health & Wellness
- Flu, COVID-19, & RSV
- Billing & Insurance
- Financial Assistance
- Medicare and MassHealth ACOs
- Participate in Research
- Educational Resources
- Visitor Information
- Find a Location
- Shuttles
- Visitor Policies
- Find Care
-
- Overview
- Our Virtual Care Options
- Virtual Urgent Care
- Virtual Visits for Primary & Specialty Care
- Online Second Opinions
-
- Overview
- Participate in Research
-
- Overview
- About Innovation
- About
- Team
- News
- For Industry
- Venture Capital and Investments
- World Medical Innovation Forum (opens link in new tab)
- Featured Licensing Opportunities
- For Innovators
- Commercialization Guide for Innovators
- Contact us
-
- Overview
- Information for Researchers
- Compliance Office
- Research Cores
- Clinical Trials
- Advisory Services
- Featured Research
- Two Centuries of Breakthroughs
- Advances in Motion (opens link in new tab)
- Brigham on a Mission (opens link in new tab)
- Gene and Cell Therapy Institute
- Research News
- Alzheimer's Disease
- Artificial Intelligence
-
- Overview
-
- Overview
- Residency & fellowship programs
- Brigham and Women's Hospital
- Massachusetts General Hospital
- Mass Eye and Ear
- Newton-Wellesley Hospital
- Salem Hospital
- Integrated Mass General Brigham Programs
- Centers of Expertise
- Global & Community Health
- Health Policy & Management
- Healthcare Quality & Patient Safey
- Medical Education
- For trainees
- Prospective trainees
- Incoming trainees
- Current trainees
- Continuing Professional Development
Utility Links
- Research and Innovation
- Centers and Programs
- Mass General Brigham AI
- About
- Digital Clinical Resource Organization
Our aim is to deliver clinically relevant evidence.
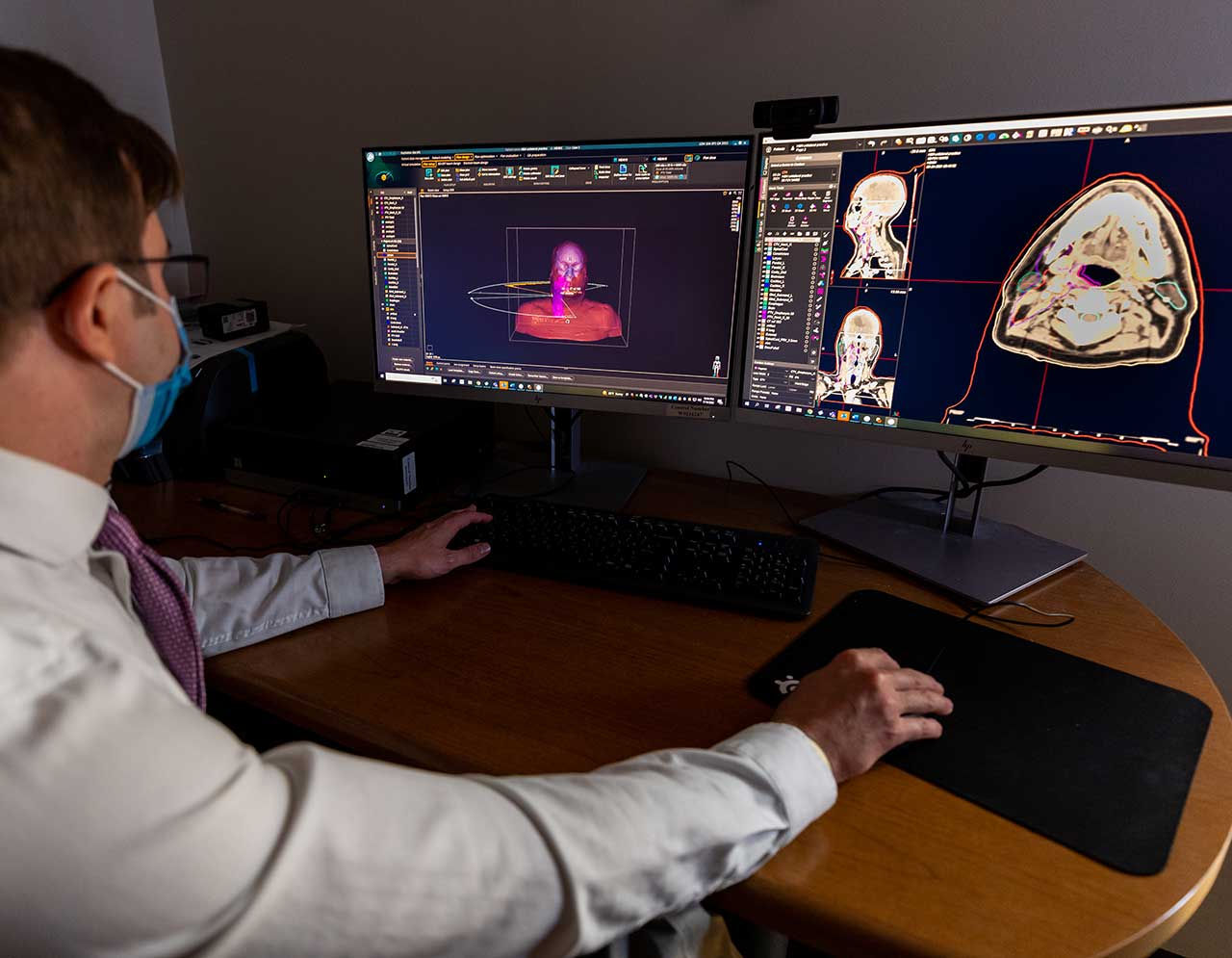
From concept to market adoption, we offer Medical and AI expertise to generate clinically relevant evidence for regulated Software as a Medical Device (SaMD) products.
Why choose us?
- Direct experience and access to clinical and image based diagnostic workflows
- Deep expertise in validation study planning, design, conduct, and reporting
- Optimal conveyance of product performance to regulators and users
- Track record of successful FDA clearance studies
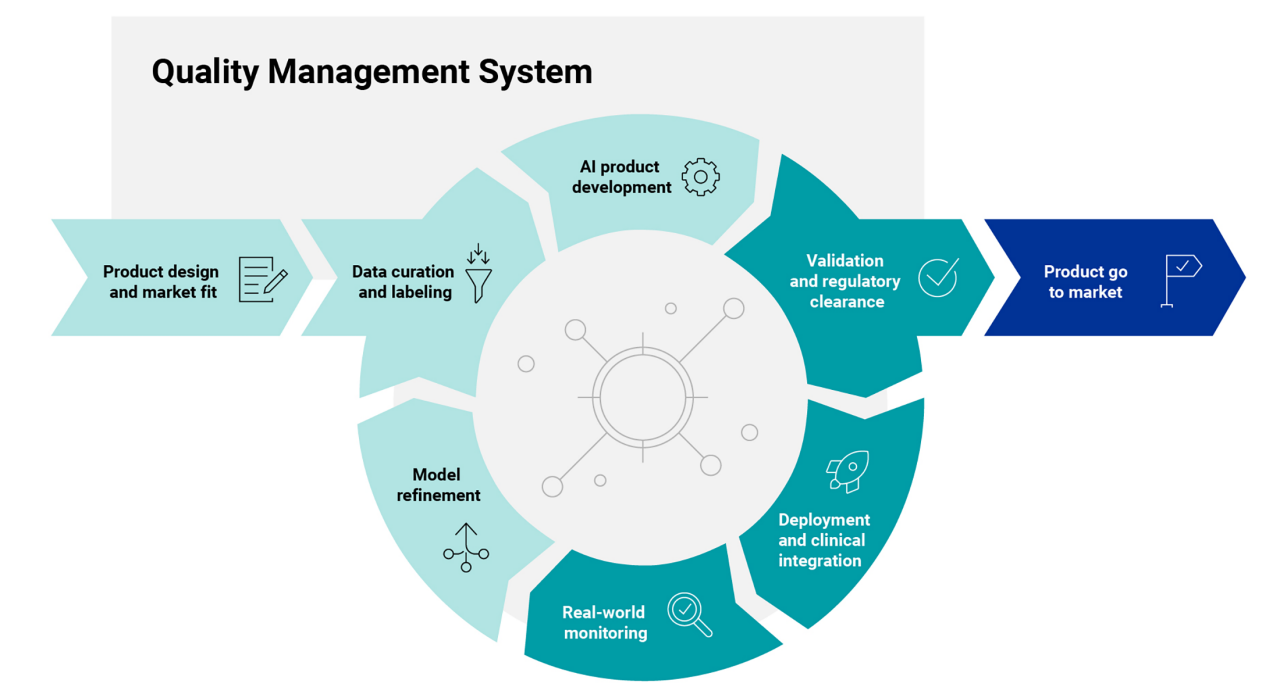
Offerings and capabilities
Validation and regulatory clearance
- Pre-submission (Q-Sub)
- Pilot and pivotal study design
- Scientific publication roadmap
- Clinical implementation strategy
- Human factors (usability) assessment
Deployment and clinical integration
- Standalone performance assessment
- Image processing/quantification
- CADt (triage)
- CADe (detection)
- CADx (diagnosis)
- Clinical reader study (CADe, CADx)
Real-world monitoring
- Ground truth and reads by U.S. board certified sub-specialty physicians
- Shadow-mode ‘real-world’ performance analysis
- Clinical workflow impact assessment
- Patient outcome and economic benefit
- Monitoring and post-market surveillance with potential for product enhancement
- Usability and human factors testing
Recent publications
Displaying %1% to %2% of %3% items
Jump to page within this carousel