United States Provisional Application: 62/909,375
WIPO PCT Application: PCT/US2020/054062
United States Patent Application: 17/765,667
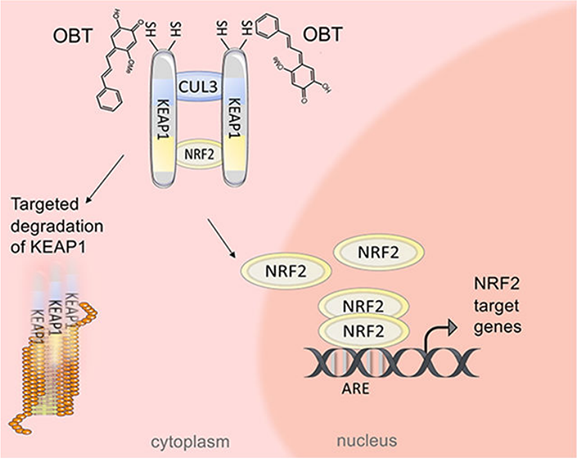
This invention is analogs to Obtusaquinone (OBT), a known compound the inventors have shown to be effective against brain tumors and breast cancer. These phenylallylidene compounds can penetrate the blood-brain barrier and target brain tumors by inducing a stress response. Uniquely, these compounds can target cancer cells while preserving normal cells.
As compared to OBT, these compounds are more stable, soluble, and potent. Since these compounds target the KEAP1/Nrf2 complex, a complex responsible for cell homeostasis and oxidative stress, they have therapeutic potential beyond cancer.
Neurodegenerative diseases, such as Alzheimer’s, ALS, and Parkinson’s, could benefit from this therapy.
Cancer therapeutics have made great strides in slowing tumor growth and spread, but the challenge remains for preserving healthy cells while targeting tumor cells. This approach targets cancer cells, can pass through the blood-brain barrier, and possibly offers a novel therapy for aggressive cancers, such as glioblastomas, and neurodegenerative diseases.
This technology is a novel potential therapy for cancer and neurological disorders. Because such therapeutics can pass through the blood-brain barrier, the potential applications are brain cancers, as well as ALS.
Bakhos A. Tannous, PhD
Dr. Tannous is now the head of NeuroOncology Discovery at AstraZeneca. Before his role at AstraZeneca, Dr. Tannous was the director of the Interdepartmental Neuroscience Center and the viral vector development facility and was codirector of the molecular neurogenetics unit-east in the neurology department at Massachusetts General Hospital. He was also an associate neuroscientist in the neurology department at Massachusetts General Hospital and was an associate professor of neurology at Harvard Medical School. His research focused on developing experimental therapeutics against pediatric and adult brain tumors, such as glioblastomas and diffuse intrinsic pontine gliomas.
David Silva, PhD
Associate Director, Business Development & Licensing, Mass General Brigham Innovation
dsilva18@mgb.org
Brain tumors and related nervous system tumors are the leading cause of cancer deaths for men under 40 years old and women under 20 years old. They also cause 26% of childhood cancer deaths. These tumors are often resistant to therapy and inaccessible for resection. Brain tumors and nervous system tumors greatly impact quality of life, as they can impact mobility, motor skills, the senses, etc.
This technology has the potential to treat neurodegenerative diseases, such as ALS, Alzheimer’s, and Parkinson’s. Alzheimer’s disease is the most common form of dementia. ALS causes progressive loss of muscle control, and Parkinson’s disease causes uncontrollable movements, such as tremors and stiffness.
Brain tumors and nervous system tumors are often treated with focalized radiation, but this treatment only stabilizes symptoms and extends median survival by three months. For children, their molecular genetics and brain chemistry is unique as they are still developing, making treatment more difficult. Alzheimer’s disease, ALS, and Parkinson’s disease are neurodegenerative diseases that have no cures.
The inventors have previously studied OBT and its anti-cancer effects in mouse models. They have also studied these OBT compounds in mouse xenograft models to understand their molecular properties and efficacy in targeting cancer cells. In these studies, the inventors found that their OBT compounds effectively led to cancer cell apoptosis and were more soluble, stable, and potent than OBT.
OBT analogs have been shown to have greater stability, potency, and solubility than OBT. These analogs also are able to penetrate the blood-brain barrier and effectively target brain tumors while preserving normal cells.
This technology could have broad applicability; it has been shown to be effective against breast cancer in mouse models and, based on its mechanism of action, could potentially be effective against neurodegenerative diseases, such as Parkinson’s, Alzheimer’s, and ALS.
Currently, there are no OBT-derived therapies on the market. These novel compounds can cross the blood-brain barrier, offering a therapy for brain cancer, notorious for poor prognoses and limited treatment options. Additionally, this novel OBT therapy can preserve normal cells while effectively targeting cancer cells.
Each year, 25,000 adults in the U.S. are diagnosed with a cancerous tumor of the brain or spinal cord. Brain cancer cases are expected to increase to 31,000 diagnosed cases in 2030. The growing aging population and exposure to radiation and air pollution are key drivers for growing brain and spinal cord cancer cases.
The global brain tumor therapeutics market is estimated to reach $2.74 billion in 2023 at a CAGR of 11% over 5 years.
In terms of neurodegenerative diseases, Alzheimer’s disease afflicts more than 6 million Americans. Alzheimer’s cases are expected to increase over time as the aging population grows. Also, more than 30,000 people in the U.S. have ALS, and more than 5,000 Americans are diagnosed with ALS each year.
Broadly, this technology has the potential to treat other neurodegenerative diseases, such as Parkinson’s Disease.
United States Provisional Application: 62/909,375
WIPO PCT Application: PCT/US2020/054062
United States Patent Application: 17/765,667