-
- Find Care
-
- Visitor Information
- Find a Location
- Shuttles
- Visitor Policies
-
-
- Our Virtual Care Options
- Virtual Urgent Care
- Virtual Visits for Primary & Specialty Care
- Online Second Opinions
- Participate in Research
-
- Contact us
-
- For Innovators
- Commercialization Guide for Innovators
-
-
- Research News
- Alzheimer's Disease
- Artificial Intelligence
-
- Overview
-
- Overview
- Getting Started
- New to Mass General Brigham
- International Patient Services
- What Is Patient Gateway?
- Planning Your Visit
- Find a Doctor (opens link in new tab)
- Appointments
- Patient Resources
- Health & Wellness
- Flu, COVID-19, & RSV
- Billing & Insurance
- Financial Assistance
- Medicare and MassHealth ACOs
- Participate in Research
- Educational Resources
- Visitor Information
- Find a Location
- Shuttles
- Visitor Policies
- Find Care
-
- Overview
- Our Virtual Care Options
- Virtual Urgent Care
- Virtual Visits for Primary & Specialty Care
- Online Second Opinions
-
- Overview
- Participate in Research
-
- Overview
- About Innovation
- About
- Team
- News
- For Industry
- Venture Capital and Investments
- World Medical Innovation Forum (opens link in new tab)
- Featured Licensing Opportunities
- For Innovators
- Commercialization Guide for Innovators
- Contact us
-
- Overview
- Information for Researchers
- Compliance Office
- Research Cores
- Clinical Trials
- Advisory Services
- Featured Research
- Two Centuries of Breakthroughs
- Advances in Motion (opens link in new tab)
- Brigham on a Mission (opens link in new tab)
- Gene and Cell Therapy Institute
- Research News
- Alzheimer's Disease
- Artificial Intelligence
-
- Overview
-
- Overview
- Residency & fellowship programs
- Brigham and Women's Hospital
- Massachusetts General Hospital
- Mass Eye and Ear
- Newton-Wellesley Hospital
- Salem Hospital
- Integrated Mass General Brigham Programs
- Centers of Expertise
- Global & Community Health
- Health Policy & Management
- Healthcare Quality & Patient Safey
- Medical Education
- For trainees
- Prospective trainees
- Incoming trainees
- Current trainees
- Continuing Professional Development
Gene Editing Tech Improves Vision
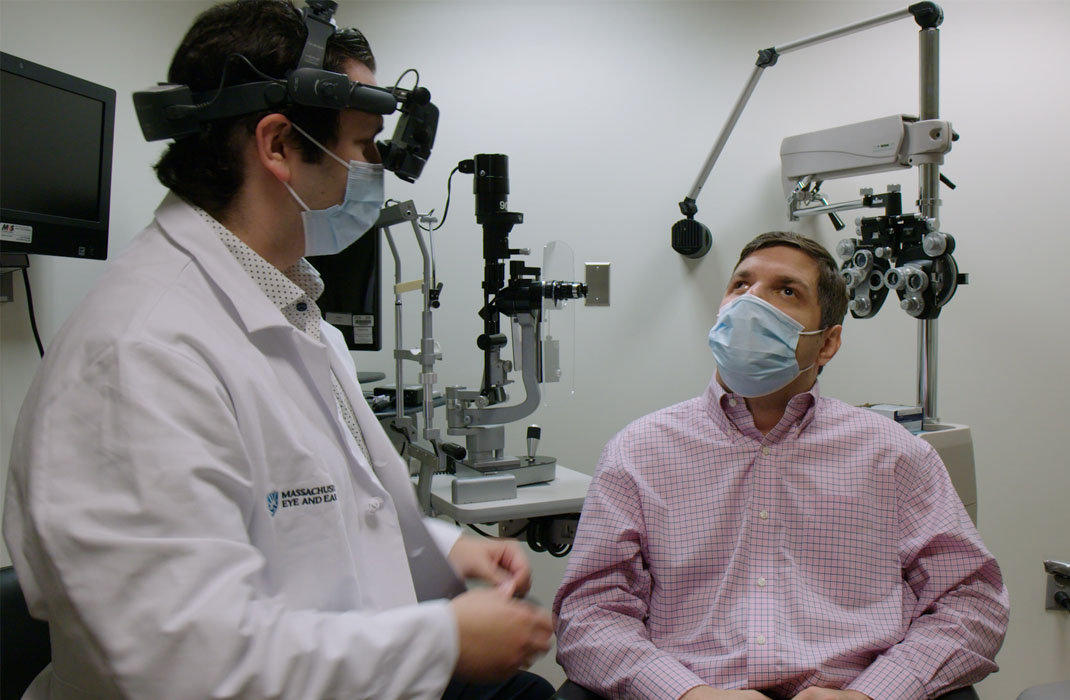
Inherited retinal degenerations (IRDs) are genetic disorders that can cause severe problems with vision, even blindness. New research is exploring the use of experimental gene-editing technology to correct faulty genes and restore vision.
In a recent study led by investigators at Mass General Brigham in collaboration with the gene editing company Editas Medicine, 11 of 14 patients with IRD achieved improvements in vision, with no serious side effects. The findings show that CRISPR gene-editing therapy is a promising avenue for future research in vision loss, and other inherited disorders.
“The results of this study provide proof of concept that CRISPR-Cas9 gene editing can be used safely and effectively to treat inherited retinal disorders,” said Eric Pierce, MD, PhD, the study’s principal investigator and director of the Ocular Genomics Institute at Mass Eye and Ear.
“While more research is needed to determine who may benefit most, we consider the early results promising. To hear from several participants how thrilled they were that they could finally see the food on their plates—that’s a big deal. These were individuals who couldn’t read any lines on an eye chart and who had no treatment options, which is the unfortunate reality for most people with inherited retinal disorders,” Dr. Pierce told CNN during a recent interview.
Inherited retinal diseases
The retina is a layer of tissue at the back of your eye. It senses light and sends signals to your brain, which translates the light into the images you see.
In some people with IRDs, errors in genes required for vision cause malfunction of the retina and serious problems with vision. Mutations in the CEP290 gene are the leading cause of severe early onset inherited retinal degeneration (CEP290-IRD), which has historically been called Leber congenital amaurosis (LCA) and often leads to severe vision loss in children.
Gene editing technology to restore vision
The phase 1/2 study, titled BRILLIANCE, included 12 adults and two children with CEP290-IRD. The researchers injected the CRISPR-Cas9 gene-editing medicine under the retina in one eye of each participant. The CRISPR-Cas9 gene editing activity removed a mutation from the CEP290 gene, restoring function to the previously inactivated gene.
Then the researchers monitored patients for up to three years to assess vision, treatment safety, and quality of life. Dr. Pierce said the treatment led to “meaningful improvement” in vision for most patients by three months. The treatment wasn’t able to fully restore vision. But some patients could see their cell phones light up, foods on their plates, the icon on a computer screen, and sunsets. The results were published in the New England Journal of Medicine.
The results offer “proof of concept,” demonstrating that CRISPR-Cas9 should be studied further in inherited blindness and other genetic diseases. The team and sponsor Editas Medicine are seeking partners to expand their work. Future goals are to conduct a phase 3 trial in a larger group of people for a longer period of follow-up.
